Sessions/ Tracks
ConferenceSeries Ltd invites all the participants from all over the world to attend “Global Surgeons Meeting on Pacemaker Implant Surgery” to be held on July 10-12, 2017 at Baltimore, USA which includes keynote presentations, oral talks, poster presentations and Exhibitions from eminent personalities from across the globe. ConferenceseriesLLC has collaborated with OMICS International for conducting scientific events.
Pacemaker Surgery 2017 provides a global platform to discuss and learn about the recent trends and approaches in the pharmacotherapy and invasive & minimally invasive surgical procedures to the problems associated with vascular diseases.
Track 1: Surgical Implantation of Pacemakers
The procedure to implant a pacemaker is usually quick. It does not require open-heart surgery, and most people go home within 24 hours. Before the surgery, medication is usually given to make you sleepy and comfortable. The procedure is performed under local anesthesia
Track 2: Minimally / Non Invasive Implantation of Pacemakers
Permanent pacemaker insertion is considered a minimally invasive procedure. Transvenous access to the heart chambers under local anesthesia is the favored technique, most commonly via the subclavian vein, the cephalic vein, or (rarely) the internal jugular vein or the femoral vein. The procedure is typically performed in a cardiac catheterization laboratory or in an operating room (OR).
The pacing generator is typically placed subcutaneously in the infraclavicular region. Occasionally, pacemaker leads are implanted surgically via a thoracotomy, and the pacing generator is placed in the abdominal area. Single-chamber and dual-chamber pacer insertion can be accomplished from either left or right pectoral sites. After appropriate sedation, the chest is prepared with an antiseptic solution, and the area is covered with sterile drapes to keep the incision area as clean as possible.
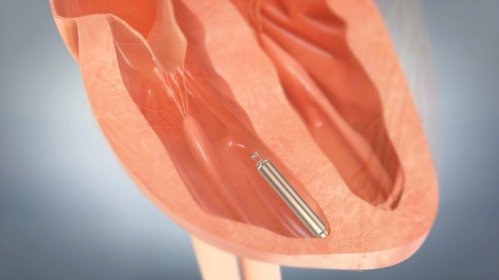
Track 3: Leadless Pacemakers
Leadless pacemaker is designed to be placed directly in the heart without the visible surgical pocket, scar and leads required for conventional pacemakers. Implanted via the femoral vein with a steerable catheter, the device offers physicians the same pacing therapy through a less-invasive approach as compared to traditional pacemaker procedures that require more extensive surgery. The device is designed to be fully retrievable, so that it can be readily repositioned throughout the implant procedure and later retrieved if necessary.
Track 4: Biventricular Pacemakers
A biventricular pacemaker is a special pacemaker used for cardiac resynchronization therapy in heart failure patients. Cardiac resynchronization therapy (CRT), also called biventricular pacing, uses a special kind of pacemaker, called a biventricular pacemaker, designed to treat the delay in heart ventricle contractions. It keeps the right and left ventricles pumping together by sending small electrical impulses through the leads. This therapy has been shown to improve the symptoms of heart failure and the person's overall quality of life.
Track 5: Clinical Trials in Pacemaker Implantation
Clinical trials are research studies that test how well new medical device or procedure work in human population. Each study answers scientific questions and tries to find better ways to prevent, screen for, diagnose, or treat a disease. Clinical trials may also compare a new treatment to a treatment that is already available.
Every pacemaker implantation clinical trial has a protocol, or action plan, for conducting the trial in testing who well the pacemaker improves the quality of life and reduce morbidity and mortality.
Track 6: Implantable Cardioverter Defibrillators (ICDs)
An implantable cardioverter defibrillator (ICD) is a small device that's placed in the chest or abdomen. ICD uses electrical pulses or shocks to help control life-threatening arrhythmias, especially those that can cause sudden cardiac arrest (SCA).
ICDs are useful in preventing sudden death in patients with known, sustained ventricular tachycardia or fibrillation (View an animation of an ICD). Studies have shown ICDs to have a role in preventing cardiac arrest in high-risk patients who haven't had, but are at risk for, life-threatening ventricular arrhythmias.
Newer-generation ICDs may have a dual function which includes the ability to serve as a pacemaker. The pacemaker feature would stimulate the heart to beat if the heart rate is detected to be too slow.
Track 7: Market of Implant Devices
The Global Market has been thoroughly scrutinized and then carefully demarcated by geographic locations which are based on major economic regions and their topographical regions. Growing competition and the changing market dynamics has been highlighted. Aggressive market players are profiled with attributes of company overview, financial overview, business strategies, product portfolio and recent developments. The Market share and Market size prominent players for 2015 are profiled in this report. The global cardiac implants market is growing at a CAGR of 9.8% from 2015 to 2020.
Track 8: Advancements in Implant Devices
Cardiac Implants and Monitoring Devices are used for not only improving the lifestyle but also extending the lifespan of millions of people worldwide. By engineering modifications, the latest changes in the last 40 years have revolutionized the treatment of cardiac diseases. Cardiac Implants and Monitoring Devices have drastically improved early diagnosis, treatment and epidemiology of various heart diseases and congenital heart ailments.
Track 9: Side Effects of Implant Device
As with any medical or surgical procedure, pacemaker implantation has risks as well as benefits. Some of the common effects include Blood clots, Pacemaker infection, Air leak, Problems with the pacemaker, Twiddler's syndrome.
About Conference
Conference series LLC invites all the participants across the world to attend ‘Global Surgeons Meeting on Pacemaker Implant Surgery’ to be held during July 10-12, 2017 Baltimore, USA which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions.
ConferenceSeries Ltd organizes 1000+ Global Events inclusive of 300+ Conferences, 500+ Workshops and 200+ Symposiums on various topics of Science & Technology across the globe with support from 1000 more scientific societies and Publishes 500+ Open Access journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Conference series LLC which organizes 1000+ Global events every year is delighted to welcome all the interested and enthusiastic participants across the globe to its prestigious Conference on Global Surgeons Meeting on Pacemaker Implant Surgery which is going to be held during July 10-12, 2017 Baltimore, USA. The theme “Pacemaker: A Push Start for Life” provides an excellent opportunity to share views, exchange knowledge and establish research collaborations & networking. The main aim of this conference is to exchange new scientific and clinical information in the field of Pacemaker Surgery bringing together among the surgeons, physicians, scientists, and other allied health professionals under one roof.
Why to attend??
Medical doctors, Surgeons, patients and health care providers are going to be gathered on a common agenda to sustain life through proper pacing of heart. Pacemakers are consider to prevent irregular pacing of heart, as an essential tool to improve the general health status of the population. The proportions of people suffering from the pacemaker dysfunction are expected to increase in future according to a recent statistical survey. According to recent statistics, heart diseases worldwide will double between 2012 and 2030. Realizing this imperative, Conference series LLC is set to organize Pacemaker Surgery 2017 this year with a view to enhance research and promote awareness aiming in developing solutions for the challenges encountered. Pacemaker Surgery-2017 will comprise of many leading keynote speakers and session speakers who will be delivering their speech on the current research topics and advances in Pacemaker Surgery, complications related to Pacemaker surgery and other risk factors associated. The young researchers and the student participants will gain the opportunity to grab the Best Poster Award by presenting their work as a poster presentation and Young Researcher Forum.
Target Audience:
· Pacemaker Implant Surgeons
· Cardiac Surgeons
· Doctors
· Research Scholars
· Scientists
· Students
· Business Delegates
· Innovators
· Investors
· Professors
· Business Entrepreneurs
· Manufacturing Medical Devices Companies
Market Analysis Report
Cardiac Implantable devices aim to treat long term heart conditions such as heart failure. These devices are intended to detect any fluctuations in the cardiac system and rectify it automatically or provide support to organs and tissues. Advancement in technology, growing incidences of cardiovascular diseases such as coronary artery diseases (CAD), arrhythmia and launch of new and innovative cardiac products such as leadless pacemakers, subcutaneous defibrillators (S-ICDs) are some of the key drivers for the growth of this market.
The market will show a significant growth in the next few years and is expected to reach $43.4 billion by 2020 at a CAGR of 12.5%. Advancement in technology, growing incidences of cardiovascular diseases such as coronary artery diseases (CAD), atrial fibrillation and aortic aneurysms and launch of new and innovative cardiac products such as leadless pacemakers, subcutaneous defibrillators (S-ICDs) are some of the key drivers for the growth of this market.

The market for Cardiac implantable devices is classified into several categories based on types of implantable devices, disease conditions and cardiac procedures. They can be utilized in the diagnosis and treatment of various disease conditions such as Myocardial Ischemia, Cardiac Arrest, Arrhythmia, Acute Myocardial Infarction and many others.
Among cardiac implantable devices, coronary stents devices are generating the largest revenue whereas in future TAVR (Transcatheter Aortic Valve Replacement) valves will capture major market segment.
Major market players are profiled along with company overview, financial status, business strategies, product portfolio, recent trends and developments.
The prominent players profiled in this report are:
· Medtronic Inc.,
· Boston Scientific Corporation
· St. Jude Medical Inc.,
· Abbott Vascular Inc.
· Edwards Lifesciences Corporation.
The report details current market potential and future market growth in this industry. Overall the report contains the most detailed and in depth segmentation and analysis of the Global Cardiac Implantable Devices Market during the forecast period 2015-2020.